This is part of Intercalation’s paid series where we dive deeper into battery companies. In this issue, we explore 24M.
Origin
24M was founded in 2010, based on a semi-solid electrode technology that spun out of work from the Yet-Ming Chiang group at MIT and A123 systems. The original idea was to create a lithium-ion redox flow battery, made with slurry suspensions of electrode materials. However, they quickly pivoted to using the semi-solid electrodes in static pouch cells after analyses showed that a Li-ion redox flow battery would be tough, even at scale. Imagine the energy losses associated with pumping concrete. Steve Levine previously wrote a great article on the early days of 24M which covered this journey.
Today the company consists of a team of 100-200 managed by CEO Naoki Ota and cofounders Throop Wilder and Yet-Ming Chiang. They have recently raised a series F round and in total have been funded north of $100M over the last decade.

So, what of the name 24M? Yet-Ming Chiang says the name is a reference to 24 molar, a concentration that is “technically significant to the company.” Here’s what we think: with lithium concentration in typical NMC materials sitting around 50 M, a slurry with 50% solids content brings us pretty close to the magic 24 M!
Anyway, onto the tech:
Semi-Solid Technology
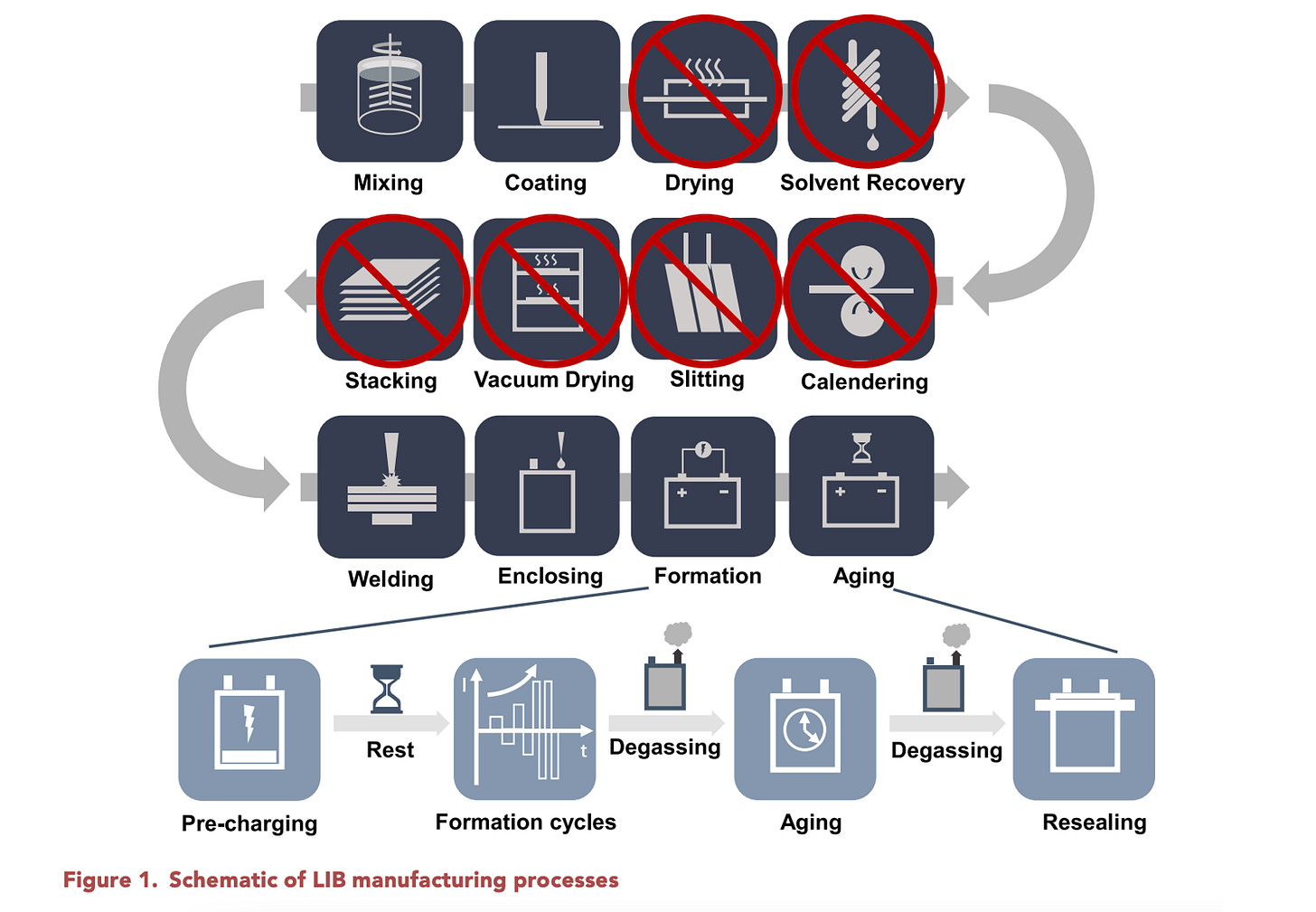
24M is commercialising its semi-solid battery technology which simplifies the production process for manufacturing lithium-ion batteries. Semi-solid electrodes are made by combining electrode powders directly with electrolytes into a clay-like mixture which is then spread onto the current-collector metal foils. The concept was originally published in 2011 when 24M was first exploring pumping these semi-solid slurries in a flow battery. This core innovation allows for several simplifications to the traditional battery architecture and manufacturing process:
By coating slurry (clay) electrodes that are 4-5 times thicker, they can reduce the amount of separator, and current collectors (copper, aluminium) used in the cell. This reduces the bill of materials cost by 20%.

The electrode powders are directly mixed into a slurry using lithium-ion electrolytes instead of the typical slurry solvents, which reduces manufacturing costs associated with drying, calendaring, and solvent recovery which also has an estimated cost saving of 20%. The
Energy consumption associated with slurry NMP solvent recovery is also significant. As electrodes are usually coated using a slurry mixed with NMP solvent instead of electrolyte, this can account for up to 46% of the energy consumption.
Finally, electrolyte filling in traditional methods is a major bottleneck, which is eliminated in this process. The traditional machinery for dosing electrolytes has been estimated in the $6-12M range according to this RWTH Aachen report. Even more process savings are gained from removing the wait time required for even liquid distribution.
Spreading the electrolyte-electrode particle slurry directly onto the current collector also should remove the cracking issues which plague electrodes as loadings increase. As normal electrodes are coated with a different solvent (water or NMP) before being completely dried, the drying process induces stresses on the coatings which cause cracks.
These thicker electrodes will however lead to slower rate capability, which is why 24M originally wanted to flow them — convection improves power capability. As elaborated on below, the initial target market for these cells seem to be for stationary storage applications
More recently, their product roadmap has consisted of dual electrolyte cells with a solid electrolyte separator, which may enable lithium metal anodes:




