Novel Operational Strategies to Enhance Battery Performance
Part 1 of optimising battery performance
How a battery performs very much depends on not just how it was made, but how it is treated. There is a lot of innovation these days making batteries last well and longer. Here are some of the existing ways to optimize battery performance, and the next two parts will cover the up and comers!
Conventional Strategies for Juicing Up Batteries
Heat ‘em up!
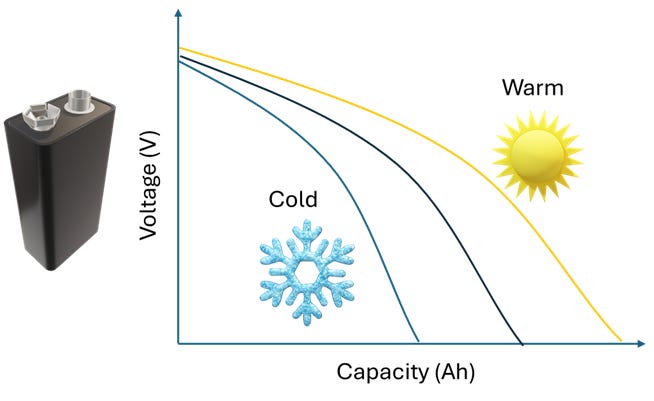
Heat has long been identified as an essential knob to fine tuning lithium-ion battery performance. Thermal management is critical to extracting max energy out of the battery while controlling the inherent degradation mechanisms. If the battery runs warm (45°C), the enhanced reaction kinetics and ion transport allow for higher energy extraction from the battery in a single discharge (imagine an EV battery in Texas in summers).
This is a double-edged sword though; both good (intercalation) and bad (solid electrolyte interphase, SEI) reactions are accelerated and if we keep the battery at high temperatures, its cycle and calendar life is diminished. Conversely, at freezing temperatures (think Alaska in winters), the kinetics/transport is incredibly sluggish; extracting energy out of the battery becomes a challenge. At the same time, all degradation reactions are also slowed, so the battery can last for tens of years if kept at such temperatures.
So, temperature control is an essential strategy to optimize the performance of any battery. To optimize charging speed in cold weather, Tesla vehicles automatically preheat the battery when you navigate to a Supercharger using the Tesla navigation system, ensuring the battery is at the optimal temperature for fast charging. Essentially, you want it hot to charge, and cold to store.
Another company EC power has developed an all-climate battery technology that enables fast charging under all conditions, including sub-freezing temperatures. The technology introduces a thin Ni foil inside the battery that forms a third terminal through which high currents (~8C) for short duration (~12-30s) are routed to provide self-heating effect from Joule heating (P = I2R) during charge/discharge at low temperatures ranging from -20°C to -40°C. This rapidly warms the cell allowing the cell surface temperature to reach near zero with a heating rate of ~1.2 °C/s while consuming around 10% of the nominal energy of the battery.
It is interesting to note that temperature directly impacts all facets of electrochemistry: thermodynamics (cell voltage at rest), reaction kinetics at the interfaces and ion transport dynamics inside the battery.
Squish ‘em up!
Another common strategy to improve battery performance is applying moderate pressure. Cell performance under pressure fixtures is enhanced due to improved electrical contact, controlling cell swelling, and preventing Li plating. Pressure on cylindrical/pouch/prismatic cells can be applied using external structures like end plates and clamping mechanisms. Sometimes, compliant materials like foam pads are placed between cells to help distribute the pressure evenly and accommodate cell expansion. At the laboratory scale, coin cells have internal wave spring and spacer assembly to keep the electrode stack under pressure. Wave springs can provide ~100 kPa pressure on the electrode stack.
Pressure primarily impacts both the interfacial reaction kinetics and ion transport inside the battery by keeping the electrode stack confined and promoting good contact between the active material and the electrolyte. This is especially important in pouch cells as exacerbated SEI reactions can evolve gases that bulge up the cells, creating more tortuous ion pathways and artificial loss of active electrochemical areas.
Generally, pressures in the hundreds of kPa to single digit MPa are recommended for operation to avoid exacerbating the cost of heavy pressure fixtures. In academic literature, this metric is generally manipulated when it comes to solid state batteries with 100s of MPa of stack pressures utilized to make solid-state cells work. The challenge is to bring this down to < 1 MPa level for practical solid-state batteries. Quantumscape’s testing of single layer cells at 3.4 atm (0.34 MPa) is a promising step in the right direction.

Pressure also influences all facets of electrochemistry: thermodynamics, reaction kinetics and transport with a more outsized role on the latter two since we are mostly concerned with low to moderate pressures in the kPa-MPa range. Thermodynamics is only mildly impacted by low pressures with GPa level stresses required to move the needle thermodynamically.
While the role of heat and pressure on impacting battery performance has been researched for decades now, new operational techniques are emerging to improve battery performance. We will focus on two of these technologies, magnetism and acoustics, in the next parts.
🌞 Thanks for reading!
📧 For tips, feedback, or inquiries - reach out
📣 For newsletter sponsorships - click here





Just to add, QuantumScape is well past the single-layer cell stage: https://www.quantumscape.com/technology/#qse-5